Mannans

Mannans are polymers containing the sugar mannose as a principal component.[1][2] They are a type of polysaccharide found in hemicellulose, a major source of biomass found in higher plants such as softwoods. These polymers also typically contain two other sugars, galactose and glucose. They are often branched (unlike cellulose).
Structural diversity
[edit]Plant mannans have β(1-4) linkages, occasionally with α(1-6) galactose branches, forming galactomannans. They are insoluble and a form of storage polysaccharide. Ivory nut is a source of mannans. An additional type is galactoglucomannan found in soft wood with a mixed mannose/glucose β(1-4) backbone.[citation needed] Conjac and salep have glucomannans with β(1-4) linkages.[3][4] Many mannans are acetylated and some from marine sources, have sulfate esters side chains.
Yeast have a different type of glucomannan in their cell wall, with a α(1-6) linked backbone and α(1-2) and α(1-3) linked glucose branches, which may also contain phosphodiester bonds.[5] Enzymatic digestion or acid catalysis can help solubilize the glucomannan.[6] It is serologically similar to structures found on mammalian glycoproteins. Detection of mannan leads to lysis in the mannan-binding lectin pathway.[citation needed]
Synthesis and degradation
[edit]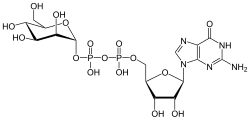
GDP-mannose is a substrate for glycosyltransferase for enzymes called mannosyltransferases.[8]
Biosynthesis
[edit]GDP-mannose is produced from GTP and mannose-6-phosphate by the enzyme mannose-1-phosphate guanylyltransferase.
The degradation of mannans (and many related forms of hemicellulose) has been well studied. The hydrolysis of the main mannan backbone is catalyzed by various enzymes including β-mannosidase, β-glucosidase, and β-mannase. The side chains are degraded by esterases and α-galactosidase.[1]
When a long chain of mannan is hydrolyzed into shorter chains, these smaller molecules are known as mannan oligosaccharide (MOS). MOS by definition can be produced from either insoluble galactomannan or soluble glucomannan, although the latter type is more widely marketed.[9]
Glucomannan MOS is used as prebiotics in animal husbandry and nutritional supplements due to its bioactivity.[citation needed]
Etymology
[edit]From 'manna', produced by several species of tree and shrub e.g. Fraxinus ornus from whose secretions mannitol was originally isolated.
See also
[edit]References
[edit]- ^ a b Moreira, L. R. S.; Filho, E. X. F. (2008). "An overview of mannan structure and mannan-degrading enzyme systems". Applied Microbiology and Biotechnology. 79 (2): 165–178. doi:10.1007/s00253-008-1423-4. PMID 18385995. S2CID 9746196.
- ^ Mannan at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ Ye S, Zongo AW, Shah BR, Li J, Li B (2021). "Konjac Glucomannan (KGM), Deacetylated KGM (Da-KGM), and Degraded KGM Derivatives: A Special Focus on Colloidal Nutrition". Journal of Agricultural and Food Chemistry. 69 (44): 12921–12932. doi:10.1021/acs.jafc.1c03647.
- ^ Kurt A, Kahyaoglu T (2017). "Purification of glucomannan from salep: Part 1. Detailed rheological characteristics". Carbohydrate Polymers. 168: 138–146. doi:10.1016/j.carbpol.2017.03.060.
- ^ Baek KR, Ramakrishnan SR, Kim SJ, Seo SO (2024). "Yeast cell wall mannan structural features, biological activities, and production strategies". Heliyon. 10 (6). doi:10.1016/j.heliyon.2024.e27896. PMC 10958358. PMID 38524613. Art. No. e27896.
- ^ de Souza Theodoro S, Putarov TC, Tiemi C, Volpe LM, de Oliveira CA, de Abreu Glória MB, Carciofi AC. "Effects of the solubility of yeast cell wall preparations on their potential prebiotic properties in dogs". PLoS One. 14 (11). doi:10.1371/journal.pone.0225659. PMC 6878821. PMID 31765439. Art. No. e0225659.
- ^ Samuel G, Reeves P (2003). "Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly". Carbohydrate Research. 338 (23): 2503–19. doi:10.1016/j.carres.2003.07.009. PMID 14670712.
- ^ Pauly, Markus; Gille, Sascha; Liu, Lifeng; Mansoori, Nasim; De Souza, Amancio; Schultink, Alex; Xiong, Guangyan (2013). "Hemicellulose biosynthesis". Planta. 238 (4): 627–642. Bibcode:2013Plant.238..627P. doi:10.1007/s00425-013-1921-1. PMID 23801299. S2CID 17501948.
- ^ Nopvichai, C; Charoenwongpaiboon, T; Luengluepunya, N; Ito, K; Muanprasat, C; Pichyangkura, R (2019). "Production and purification of mannan oligosaccharide with epithelial tight junction enhancing activity". PeerJ. 7 e7206. doi:10.7717/peerj.7206. PMC 6611449. PMID 31304065.
MOS is often prepared by hydrolysis reaction of a mannose-contained glucan polymer, mainly glucomannan and galactomannan.
Further reading
[edit]- Stewart, James; Curtis, Joan; Spurck, Timothy P.; Ilg, Thomas; Garami, Attila; Baldwin, Tracey; Courret, Nathalie; McFadden, Geoffrey I.; Davis, Antony; Handman, Emanuela (July 2005). "Characterisation of a Leishmania mexicana knockout lacking guanosine diphosphate-mannose pyrophosphorylase". International Journal for Parasitology. 35 (8): 861–873. doi:10.1016/j.ijpara.2005.03.008. PMID 15936761.
