2,5-二甲氧基苯乙胺衍生物
外观

2,5-二甲氧基苯乙胺衍生物,也称为2C类化合物(英語:2C-x)或2C家族(英語:2C family)是苯乙胺衍生物中甲氧基苯乙胺衍生物的一类,苯基上2号位与5号位的氢被甲氧基取代,即以2,5-二甲氧基苯乙胺(也称为2C-H)为母结构,其中绝大多数的取代基位于4号位,少数位于3号位,6号位基本上没有取代基[1]。许多这一类化合物由亚历山大·舒尔金在1970年代到1980年代首次合成[2]。
衍生物
[编辑]| 名称 | R3基团 | R4基团 | R6基团 | 分子式 | 2D结构 | CAS号 |
|---|---|---|---|---|---|---|
| 2C-B | H | Br | H | C 10H 14BrNO 2 |
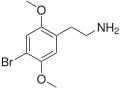
|
66142-81-2 |
| 2C-Bn | H | CH2C6H5 | H | C17H21NO2 | 
|
2888537-43-5 |
| 2C-Bu | H | CH2CH2CH2CH3 | H | C14H23NO2 | 
|
2888537-44-6 |
| 2C-C | H | Cl | H | C10H14ClNO2 | 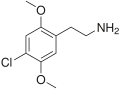
|
88441-14-9 |
| 2C-C-3 [3] | Cl | Cl | Cl | C10H12Cl3NO2 | 
|
1112937-89-9 |
| 2C-CN | H | C≡N | H | C11H14N2O2 | 
|
88441-07-0 |
| 2C-cP | H | C3H5 | H | C13H19NO2 | 
|
2888537-46-8 |
| 2C-CPE | H | C5H9 | H | C15H23NO2 | 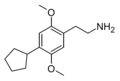
|
|
| 2C-D | H | CH3 | H | C11H17NO2 | 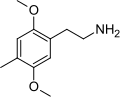
|
24333-19-5 |
| 2C-E | H | CH2CH3 | H | C12H19NO2 | 
|
71539-34-9 |
| 2C-EF | H | CH2CH2F | H | C12H18FNO2 | 
|
1222814-77-8 |
| 2C-F | H | F | H | C10H14FNO2 | 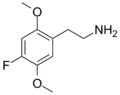
|
207740-15-6 |
| 2C-G | CH3 | CH3 | H | C12H19NO2 | 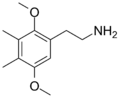
|
207740-18-9 |
| 2C-G-1 | CH2 | H | C11H15NO2 | 
|
2888537-47-9 | |
| 2C-G-2 | (CH2)2 | H | C12H17NO2 | 
|
2888537-48-0 | |
| 2C-G-3 | (CH2)3 | H | C13H19NO2 | 
|
207740-19-0 | |
| 2C-G-4 | (CH2)4 | H | C14H21NO2 | 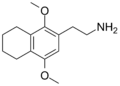
|
952006-59-6 | |
| 2C-G-5 | (CH2)5 | H | C15H21NO2 | 
|
207740-20-3 | |
| 2C-G-6 | (CH2)6 | H | C16H23NO2 | 
|
2888537-49-1 | |
| 2C-G-N | (CH)4 | H | C14H17NO2 | 
|
207740-21-4 | |
| 2C-H | H | H | H | C10H15NO2 | 
|
3600-86-0 |
| 2C-I | H | I | H | C10H14INO2 | 
|
69587-11-7 |
| 2C-iBu | H | CH2CH(CH3)2 | H | C14H23NO2 | 
|
2504100-82-5 |
| 2C-iP | H | CH(CH3)2 | H | C13H21NO2 | 
|
1498978-47-4 |
| 2C-tBu | H | C(CH3)3 | H | C14H23NO2 | 
|
PubChem CID:117347542 |
| 2C-N | H | NO2 | H | C10H14N2O4 | 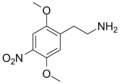
|
261789-00-8 |
| 2C-NH2 | H | NH2 | H | C10H16N2O2 | 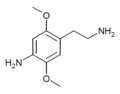
|
168699-66-9 |
| 2C-PYR | H | 吡咯烷基 | H | C14H22N2O2 | 
|
910381-23-6 |
| 2C-PIP | H | 哌啶基 | H | C15H24N2O2 | 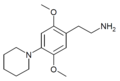
|
1898118-63-2 |
| 2C-O | H | OCH3 | H | C11H17NO3 | 
|
15394-83-9 |
| 2C-O-4 | H | OCH(CH3)2 | H | C13H21NO3 | 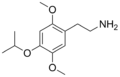
|
952006-65-4 |
| 2C-MOM[4] | H | CH2OCH3 | H | C12H19NO3 | 
|
1898203-98-9 |
| 2C-P | H | CH2CH2CH3 | H | C13H21NO2 | 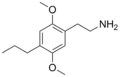
|
207740-22-5 |
| 2C-Ph | H | C6H5 | H | C16H19NO2 | 
|
1217170-12-1 |
| 2C-Se | H | SeCH3 | H | C11H17NO2Se | 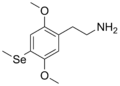
|
1189246-68-1 |
| 2C-Se-TFM | H | SeCF3 | H | C11H14F3NO2Se | 
|
|
| 2C-T | H | SCH3 | H | C11H17NO2S | 
|
61638-09-3 |
| 2C-Te | H | TeCH3 | H | C11H17NO2Te | ||
| 2C-DFM [5]:770 | H | CHF2 | H | C11H15F2NO2 | 
|
1891474-10-4 |
| 2C-TFM | H | CF3 | H | C11H14F3NO2 | 
|
159277-08-4 |
| 2C-TFE | H | CH2CF3 | H | C12H16F3NO2 | 
|
2888537-56-0 |
| 2C-PFE | H | CF2CF3 | H | C12H14F5NO2 | 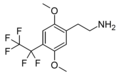
|
暂未注册 |
| 2C-PFS | H | SF5 | H | C10H14F5NO2S | 
|
暂未注册 |
| 2C-YN | H | C≡CH | H | C12H15NO2 | 
|
752982-24-4 |
| 2C-V | H | CH=CH2 | H | C12H17NO2 | 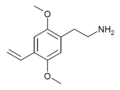
|
2888537-57-1 |
| 2C-AL[6] | H | CH2CH=CH2 | H | C13H19NO2 | 
|
2756686-02-7 |
含硫衍生物
[编辑]| 名称 | R3基团 | R4基团 | R6基团 | 分子式 | 2D结构 | CAS号 |
|---|---|---|---|---|---|---|
| 2C-T | H | SCH3 | H | C11H17NO2S | 
|
61638-09-3 |
| 2C-T-2 | H | SCH2CH3 | H | C12H19NO2S | 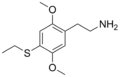
|
207740-24-7 |
| 2C-T-3[7] | H | SCH2C(=CH2)CH3 | H | C14H21NO2S | 
|
648957-40-8 |
| 2C-T-4 | H | SCH(CH3)2 | H | C13H21NO2S | 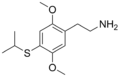
|
207740-25-8 |
| 2C-T-5[7] | H | SCH(CH2)5 | H | C16H25NO2S | 
|
1187859-38-6 |
| 2C-T-6[7] | H | SC6H5 | H | C16H19NO2S | 
|
2888537-50-4 |
| 2C-T-7 | H | S(CH2)2CH3 | H | C13H21NO2S | 
|
207740-26-9 |
| 2C-T-8 | H | SCH2CH(CH2)2 | H | C14H21NO2S | 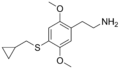
|
207740-27-0 |
| 2C-T-9[7] | H | SC(CH3)3 | H | C14H23NO2S | 
|
207740-28-1 |
| 2C-T-10[7] | H | H | 
|
2888537-51-5 | ||
| 2C-T-11[7] | H | H | C16H18BrNO2S | 
|
1798390-41-6 | |
| 2C-T-12[7] | H | H | 
|
2888537-52-6 | ||
| 2C-T-13 | H | S(CH2)2OCH3 | H | C13H21NO3S | 
|
207740-30-5 |
| 2C-T-14[7] | H | S(CH2)2SCH3 | H | C13H21NO2S2 | 
|
暂未注册 |
| 2C-T-15 | H | SCH(CH2)2 | H | C13H19NO2S | 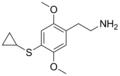
|
952006-95-0 |
| 2C-T-16[8] | H | SCH2CH=CH2 | H | C13H19NO2S | 
|
648957-42-0 |
| 2C-T-17 | H | SCH(CH3)CH2CH3 | H | C14H23NO2S | 
|
207740-32-7 |
| 2C-T-18[7] | H | SCH(CH2)3 | H | C14H21NO2S | 
|
2888537-53-7 |
| 2C-T-19 | H | SCH2CH2CH2CH3 | H | C14H23NO2S | 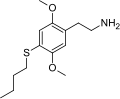
|
732244-33-6 |
| 2C-T-21 | H | S(CH2)2F | H | C12H18FNO2S | 
|
207740-33-8 |
| 2C-T-21.5[7] | H | SCH2CHF2 | H | C12H17F2NO2S | 
|
648957-46-4 |
| 2C-T-22[7] | H | SCH2CF3 | H | C12H16F3NO2S | 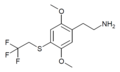
|
648957-48-6 |
| 2C-T-23[7] | H | SCH(CH2)4 | H | C15H23NO2S | 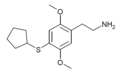
|
2888537-54-8 |
| 2C-T-24[7] | H | H | 
|
暂未注册 | ||
| 2C-T-25[7] | H | SCH2CH(CH3)2 | H | C14H23NO2S | 
|
740797-11-9 |
| 2C-T-27[7] | H | SCH2C6H5 | H | C17H21NO2S | 
|
648957-52-2 |
| 2C-T-28[7] | H | S(CH2)3F | H | C13H20FNO2S | 
|
648957-54-4 |
| 2C-T-29 | H | SCH2C≡CH | H | C13H17NO2S | 
|
|
| 2C-T-30[7] | H | S(CH2)4F | H | C14H22FNO2S | 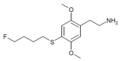
|
775578-10-4 |
| 2C-T-31[7] | H | H | 
|
765269-48-5 | ||
| 2C-T-32[7] | H | H | 
|
737754-27-7 | ||
| 2C-T-33[7] | H | H | C18H23NO3S | 
|
暂未注册 | |
| 2C-T-DFM (2C-T-35) |
H | SCHF2 | H | C11H15F2NO2S | 
|
暂未注册 |
| CYB210010 (2C-T-TFM、2C-T-36) |
H | SCF3 | H | C11H14F3NO2S | 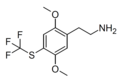
|
2762567-99-5 |
苯并杂环衍生物
[编辑]| 名称 | R3基团 | R4基团 | R6基团 | 分子式 | 2D结构 | CAS号 |
|---|---|---|---|---|---|---|
| 2C-B-FLY | 苯并二氢呋喃 | Br | 苯并二氢呋喃 | C12H14BrNO2 | 
|
733720-95-1 |
| 2C-B-BFLY | 苯并二氢吡喃 | Br | 苯并二氢吡喃 | C14H18BrNO2 | 
|
502659-24-7 |
| 2C-B-DFLY | 苯并呋喃 | Br | 苯并呋喃 | C12H10BrNO2 | 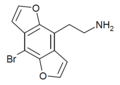
|
260809-98-1 |
| 2C-C-FLY | 苯并二氢呋喃 | Cl | 苯并二氢呋喃 | C12H14ClNO2 | 
|
1354633-83-2 |
| 2C-D-FLY | 苯并二氢呋喃 | CH3 | 苯并二氢呋喃 | C13H17NO2 | 
|
1354634-07-3 |
| 2C-E-FLY | 苯并二氢呋喃 | CH2CH3 | 苯并二氢呋喃 | C14H19NO2 | 
|
2697190-39-7 |
| 2C-EF-FLY | 苯并二氢呋喃 | CH2CH2F | 苯并二氢呋喃 | C14H18FNO2 | 
|
|
| 2C-I-FLY | 苯并二氢呋喃 | I | 苯并二氢呋喃 | C12H14INO2 | 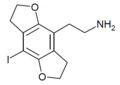
|
1354633-88-7 |
| 2C-T-7-FLY | 苯并二氢呋喃 | SCH2CH3 | 苯并二氢呋喃 | C15H21NO2S | 
|
1354633-05-8 |
参考文献
[编辑]- ^ Alexander Shulgin, Tania Manning and Paul F Daley. The Shulgin Index. Volume 1. Psychedelic Phenethylamines and Related Compounds. Transform Press, 2011. ISBN 978-0-9630096-3-0
- ^ Daniel Trachsel, David Lehmann and Christoph Enzensperger. Phenethylamine Von der Struktur zur Funktion, pp 762-810. Nachtschatten Verlag AG, 2013. ISBN 978-3-03788-700-4
- ^ Takahashi, Misako; Nagashima, Machiko; Suzuki, Jin; Seto, Takako; Yasuda, Ichirou; Yoshida, Takemi. Creation and application of psychoactive designer drugs data library using liquid chromatography with photodiode array spectrophotometry detector and gas chromatography–mass spectrometry. Talanta. 2009-02, 77 (4): 1245–1272. doi:10.1016/j.talanta.2008.07.062.
- ^ Leth-Petersen, Sebastian; Petersen, Ida N.; Jensen, Anders A.; Bundgaard, Christoffer; Bæk, Mathias; Kehler, Jan; Kristensen, Jesper L. 5-HT 2A /5-HT 2C Receptor Pharmacology and Intrinsic Clearance of N -Benzylphenethylamines Modified at the Primary Site of Metabolism. ACS Chemical Neuroscience. 2016-11-16, 7 (11): 1614–1619. doi:10.1021/acschemneuro.6b00265.
- ^ Daniel Trachsel; David Lehmann & Christoph Enzensperger. Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. 2013. ISBN 978-3-03788-700-4.
- ^ Kruegel AC. Phenalkylamines and Methods of Treating Mood Disorders. Patent WO 2022/006186 (PDF). [2023-11-19]. (原始内容存档 (PDF)于2022-12-20).
- ^ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 7.18 7.19 Shulgin's Sulfur Symphony – Part I. countyourculture. 15 January 2011 [22 October 2017]. (原始内容存档于19 September 2019).
- ^ Daniel Trachsel. Synthesis of novel (phenylalkyl)amines for the investigation of structure-activity relationships. Part 2. 4-Thio-substituted [2-(2,5-dimethoxyphenyl)ethyl]amines (=2,5-dimethoxybenzeneethanamines). Helvetica Chimica Acta. 2003, 86 (7): 2610–2619. doi:10.1002/hlca.200390210.
